
 ImmuCell Corporation (NasdaqCM: ICCC), a growing animal health company that is developing, manufacturing and selling products that improve animal health and productivity in the dairy and beef industries, today announced financial results for its first quarter ended March 31, 2014.
ImmuCell Corporation (NasdaqCM: ICCC), a growing animal health company that is developing, manufacturing and selling products that improve animal health and productivity in the dairy and beef industries, today announced financial results for its first quarter ended March 31, 2014.First Quarter Overview:
- Company reports first quarter product sales of $2.08 million, an increase of 13% compared to the same period a year ago;
- Seventh (7th) consecutive quarter of positive sales growth and thirteenth (13th) quarter of positive sales growth out of the last 14 quarters;
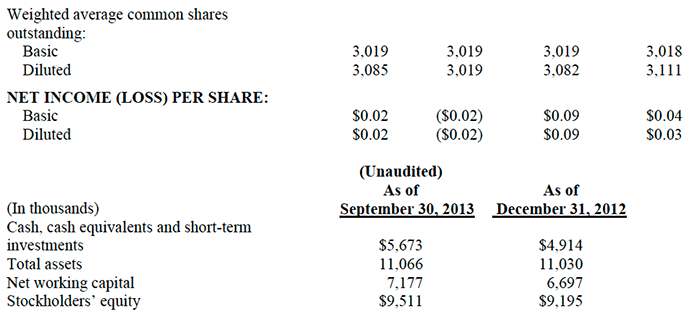
- Cash, cash equivalents, short-term and long-term investments balance at March 31, 2014 was $5.47 million;
- Approximately $412,000 in product development expenses incurred during the first quarter related to modifications to its facility to fulfill Mast Out® regulatory requirements for the manufacture of Nisin and to verify production costs; and
- Significant progress made towards Company's objective of achieving New Animal Drug Application (NADA) approval and test marketing of Mast Out® by early 2016.
Quarterly Results
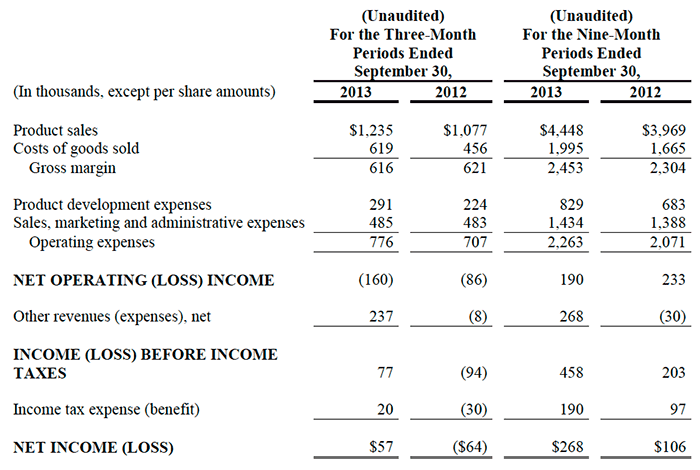
For the first quarter ended March 31, 2014, ImmuCell reported a product sales increase of 13% to $2.08 million versus $1.85 million in the comparable period of the prior year. The Company has now achieved positive sales growth for seven consecutive quarters and for thirteen out of the last fourteen quarters. Sales during the quarter were primarily driven by continued market acceptance of First Defense® for the prevention of calf scours. Also, during the second half of 2013, production had been slowed in order to upgrade certain pieces of critical manufacturing equipment. The Company ended the first quarter with approximately $155,000 of orders that were placed by customers but could not be filled by the Company before April 1, 2014. The Company is now rebuilding inventory back to historical levels with production back at full and increased capacity. Had the Company been able to fulfill all the orders placed during the first quarter in accordance with its normal shipping schedule, sales would have grown by 21% during the quarter as compared to the first quarter of 2013.
Gross margin during the first quarter of 2014 was 55%, compared to 57% in the first quarter of last year. In contrast, the gross margin was 44% during the six-month period ended December 31, 2013. Gross margin declined below normal levels during the second half of 2013 as production was slowed to replace and upgrade critical manufacturing equipment. The Company expects margins to be maintained in line with current levels throughout 2014.
Sales, marketing and administrative expenses for the first quarter were $543,000 compared to $460,000 during the same period last year. Sales and marketing expenses aggregated 14% and 12% of product sales during the first quarters of 2014 and 2013, respectively. The annual target for these expenses is up to 20% of product sales, but this percentage tends to be lower during the first quarter when seasonal sales are highest.
Product development expenses of $594,000 during the three-month period ended March 31, 2014 were comprised of $412,000 in connection with the installation of its pharmaceutical-grade Nisin production capability (which management considers to be non-recurring, infrequent and unusual expenses) and $182,000 in connection with other product development expenses.
If the Company had elected not to incur the $412,000 in non-recurring, infrequent and unusual product development expenses described above, Net Operating Income during the first quarter of 2014 would have increased from $13,000 to $425,000. This adjusted figure compares favorably to Net Operating Income of $327,000 during the first quarter of 2013. The Net (Loss) was ($13,000), or ($0.004) per share, during the first quarter of 2014, in contrast to Net Income of $204,000, or $0.07 per diluted share, during the first quarter of 2013.
Net Operating Income of $13,000 during the first quarter of 2014 included $113,000 of non-cash depreciation and amortization expenses. In comparison, Net Operating Income of $327,000 during the first quarter of 2013 included $98,000 of non-cash depreciation and amortization expenses.
Outlook
Based upon the evaluation of information currently available to management, the Company is expecting to see continued growth in product sales throughout 2014. As previously communicated, the Company has made the decision to pursue regulatory approval of Mast Out® and demonstrate its commercial viability and market acceptance on a smaller scale without a partner. After completing the method transfer to the U.S. Food and Drug Administration (FDA) laboratory, the Company expects that the Human Food Safety (HFS) Technical Section Complete Letter will be issued after a six-month review period by the FDA. The Company expects to make the first submission of the Chemistry, Manufacturing and Controls (CMC) Technical Section to the FDA at the end of 2014 or in early 2015, which will likely be followed by two, six-month review periods by the FDA. As a result, the Company hopes to be in position to achieve NADA approval and to test market the product by early 2016.
Management Discussion
Michael Brigham, president and chief executive officer of ImmuCell Corporation, commented, "2014 is shaping up to be an exciting year for ImmuCell as we execute on two very significant components of our business strategy: expand the market penetration of our best-in-class treatment for calf scours; and advance the development for FDA approval of Mast Out® for the treatment of subclinical mastitis in lactating dairy cows. We made strong progress on both fronts during the first quarter as we achieved increased product sales, driven by continued acceptance of First Defense®, and we made significant advances towards completing the modifications to our manufacturing facilities necessary to produce batches of pharmaceutical-grade Nisin required to complete the CMC Technical Section for Mast Out® approval. It remains our goal to make the first submission of the CMC Technical Section to the FDA at the end of 2014 or in early 2015."
Mr. Brigham expanded on the results of the quarter, "Sales of First Defense® increased by 7% during the quarter compared to the first quarter of 2013 due to the success of a number of management initiatives, as well as improving market conditions. Had we been able to ship all orders received but held pending regulatory lot releases (approximately $155,000) as of March 31, 2014, sales of First Defense® would have increased by 16% during the first quarter of 2014 compared to the first quarter of 2013. We continue to invest in our sales and marketing team with the addition of additional sales representatives to develop territories where our historical presence has been minimal, notably the southern portion of the United States. We also focused our efforts on expanding the market opportunity for First Defense® to include beef cattle in addition to dairy cows through a series of direct mailing campaigns. These types of initiatives, coupled with an improved milk-to-feed ratio and severe winter conditions during the first quarter of 2014 contributed to our strong sales growth."
Mr. Brigham added, "We are experiencing positive sales momentum, which I expect will continue to result in continued revenue growth throughout 2014. Additionally, with the other primary components for FDA approval achieved, we will be taking the necessary steps, and making the requisite investment, to introduce Mast Out® to the market by focusing on the manufacturing requirements for FDA approval. Mast Out® has the potential to revolutionize the way that mastitis is treated by making earlier treatment of subclinically infected cows economically feasible by not requiring a milk discard during, or for a period of time after, treatment. No other product presently on the market can offer this value proposition."
Mr. Brigham concluded, "We are confident in our ability to take advantage of the opportunities ahead of us. The team at ImmuCell is excited about what can be accomplished in 2014 and beyond to expand our business and to enhance stockholder value."
About ImmuCell
ImmuCell Corporation's (NasdaqCM: ICCC) purpose is to create scientifically-proven and practical products that improve animal health and productivity in the dairy and beef industries. ImmuCell has developed products that provide significant, immediate immunity to newly born dairy and beef livestock. The Company has also developed products that address mastitis, the most significant cause of economic loss to the dairy industry. Press releases and other information about the Company are available at our newly updated web-site, (http://www.immucell.com).
5.14.2014