
DRAXXIN portfolio expands to offer more treatment options
Zoetis today announced the launch of DRAXXIN® 25 (tulathromycin injection) Injectable Solution, a lower concentration of DRAXXIN® (tulathromycin) Injectable Solution, to treat bovine respiratory disease (BRD) in suckling, dairy and veal calves.
Just like the current concentration of DRAXXIN, DRAXXIN 25 offers broad-spectrum coverage against the major causes of BRD, including Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis. However, producers and veterinarians should note that DRAXXIN 25 has a pre-slaughter withdrawal time of 22 days, compared with the 18-day pre-slaughter time for DRAXXIN.
"The efficacy of DRAXXIN has made it a leading choice by veterinarians and producers for the treatment of BRD in larger, ruminating calves," said Dr. Robert Lynch, senior veterinarian, Dairy Technical Services, Zoetis. "The new formulation of DRAXXIN 25 allows for more-accurate and convenient dosing for smaller calves. Producers and veterinarians can be confident they are administering the correct treatment needed."
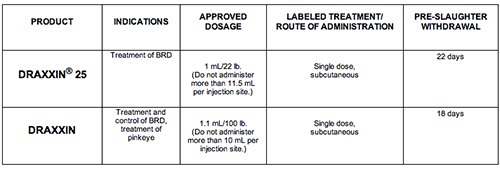
PRODUCT INDICATIONS APPROVED DOSAGE LABELED TREATMENT/ ROUTE OF ADMINISTRATION PRE-SLAUGHTER WITHDRAWAL
DRAXXIN® 25 Treatment of BRD 1 mL/22 lb. (Do not administer more than 11.5 mL per injection site.) Single dose, subcutaneous 22 days
DRAXXIN Treatment and control of BRD, treatment of pinkeye 1.1 mL/100 lb. (Do not administer more than 10 mL per injection site.) Single dose, subcutaneous 18 days
Effectively treat BRD so calves get a healthy start
DRAXXIN 25 and DRAXXIN both offer a convenient full course of therapy in a single dose. When administered according to the label, DRAXXIN is absorbed and distributed quickly to provide effective, single-dose therapy for BRD.
DRAXXIN 25 is available in 100 mL and 250 mL vials and may be purchased through your veterinarian or animal health retailer with a veterinarian's prescription.
DRAXXIN label expanded for suckling and veal calves
Zoetis also announced the approval of DRAXXIN for the treatment against the major causes of BRD associated with M. haemolytica, P. multocida, H. somni and M. bovis in suckling, dairy and veal calves. Previously, DRAXXIN was approved only for use in nonlactating dairy cattle (cattle younger than 20 months of age, including dairy calves) and beef cattle.
"Through Zoetis's efforts to obtain FDA approval to remove the veal calf restriction and include suckling calves in the DRAXXIN label, veterinarians can prescribe DRAXXIN with confidence and producers can practice responsible antibiotic use while treating BRD symptoms with a demonstrated and effective BRD treatment option for veal calves," said Dr. Lynch.
Dr. Lynch encourages veterinarians and producers to work together to establish proper treatment protocols and review BRD management practices in their calf wellness program, including:
- Pathogen identification
- BRD vaccination
- BRD symptom identification
- Record keeping
Learn more about DRAXXIN® 25 and the expanded label for DRAXXIN at Draxxin.com/Dairy or by contacting your veterinarian or Zoetis representative.
IMPORTANT SAFETY INFORMATION FOR DRAXXIN 25: DRAXXIN 25 has a pre-slaughter withdrawal time of 22 days in calves. Do not use in ruminating cattle. Do not use in animals known to be hypersensitive to the product. See full Prescribing Information.
IMPORTANT SAFETY INFORMATION FOR DRAXXIN: DRAXXIN has a pre-slaughter withdrawal time of 18 days. Do not use in female dairy cattle 20 months of age or older. Do not use in animals known to be hypersensitive to the product. See full Prescribing Information.
 About Zoetis
About Zoetis Zoetis (zo-EH-tis) is the leading animal health company, dedicated to supporting its customers and their businesses. Building on more than 60 years of experience in animal health, Zoetis discovers, develops, manufactures and markets veterinary vaccines and medicines, complemented by diagnostic products and genetic tests and supported by a range of services. In 2013, the company generated annual revenues of $4.6 billion. With approximately 9,800 employees worldwide at the beginning of 2014, Zoetis has a local presence in approximately 70 countries, including 27 manufacturing facilities in 10 countries. Its products serve veterinarians, livestock producers and people who raise and care for farm and companion animals in 120 countries. For more information, visit www.zoetisUS.com.
1.21.2015