
Producers and veterinarians now have approved Zoetis products for AI synchronization
 Dairy producers can improve the reproductive performance of their herds with approved products from Zoetis for fixed-time artificial insemination. The Food and Drug Administration (FDA) has approved FACTREL(R) (gonadorelin hydrochloride) Sterile Solution for use with LUTALYSE(R) (dinoprost tromethamine) Sterile Solution to synchronize estrous cycles to allow for fixed-time artificial insemination in lactating dairy cows.
Dairy producers can improve the reproductive performance of their herds with approved products from Zoetis for fixed-time artificial insemination. The Food and Drug Administration (FDA) has approved FACTREL(R) (gonadorelin hydrochloride) Sterile Solution for use with LUTALYSE(R) (dinoprost tromethamine) Sterile Solution to synchronize estrous cycles to allow for fixed-time artificial insemination in lactating dairy cows."We understand that reproductive synchronization is critical to the success of modern dairy operations," says John Lee, DVM, managing veterinarian, Zoetis. "The FDA's approval provides veterinarians and producers the opportunity to feel confident using products that they have trusted for years."
AI synchronization programs have grown in popularity since their introduction via university research in the 1990s. FDA approval of FACTREL for use with LUTALYSE includes a flexible schedule, allowing veterinarians to customize breeding programs for their dairy clients. Below are three examples of the flexible treatment regimen:
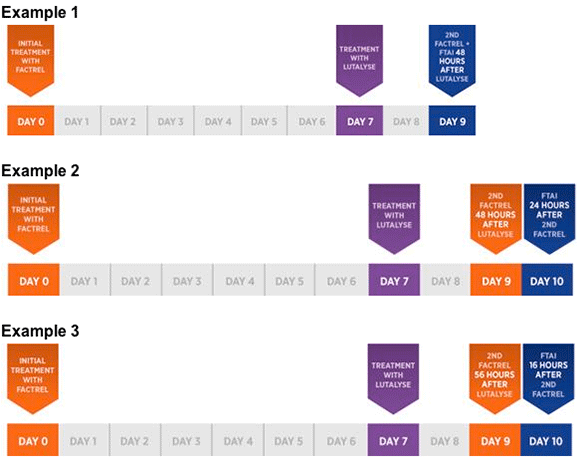
"The nice part about the approval is the flexibility it offers. Veterinarians will be able to tailor fixed-time artificial insemination programs to the specific needs of their clients while still being on label," Dr. Lee says. "At Zoetis, we consider it our responsibility to ensure the industry has access to comprehensive, on-label reproductive management solutions consistent with best practices of dairy management."
With flexible, approved use of Zoetis products for fixed-time artificial insemination, veterinarians and producers now can use LUTALYSE, the most veterinarian-recommended and producer-used prostaglandin1 in the marketplace, on label with FACTRELin many of the synchronization protocols recommended by the Dairy Cattle Reproduction Council.
To learn more about the use of FACTREL with LUTALYSE, contact your veterinarian or Zoetis representative.
Important Safety Information for FACTREL: FACTREL is available through veterinary prescription only and not for use in humans. As with all drugs, FACTREL should not be used in animals found to be hypersensitive to the product. Full prescribing information is available here.
Important Safety Information for LUTALYSE: As with all parenteral products, aseptic technique should be used to reduce the possibility of post-injection bacterial infections. Do not administer in pregnant animals unless cessation of pregnancy is desired. Not for intravenous administration. Women of childbearing age and persons with respiratory problems should exercise extreme caution when handling this product. Full prescribing information is available here.
About Zoetis
Zoetis (zo-EH-tis) is the leading animal health company, dedicated to supporting customers and businesses focused on raising and caring for livestock and companion animals. Building on a 60-year history as the animal health business of Pfizer Inc., Zoetis discovers, develops, manufactures and markets veterinary vaccines and medicines, complemented by diagnostic products and genetic tests and supported by a range of services. The company generated annual revenues of $4.3 billion in 2012. It has more than 9,300 employees worldwide and a local presence in approximately 70 countries, including 29 manufacturing facilities in 11 countries. Its products serve veterinarians, livestock producers and people who raise and care for livestock and companion animals in 120 countries. For more information, visit zoetisUS.com.
7.3.2013