The authors are with Quality Milk Production Services, Cornell University.
The frequency and cause of clinical mastitis may differ considerably between dairy herds. Therefore, descriptive results from epidemiological studies characterizing clinical mastitis can provide insight to control, treat, and prevent further cases.
Although several studies have reported the association between pathogen and actual clinical mastitis, few have prospectively monitored clinical mastitis on farms for an extended time. Quality Milk Production Services at Cornell University in New York offers a service where couriers pick up milk samples 360 days per year and return pathogen results the next day for mastitis management decisions, including treatment. This allows us to describe pathogen distribution and the true incidence risk of clinical mastitis.
One year of tracking
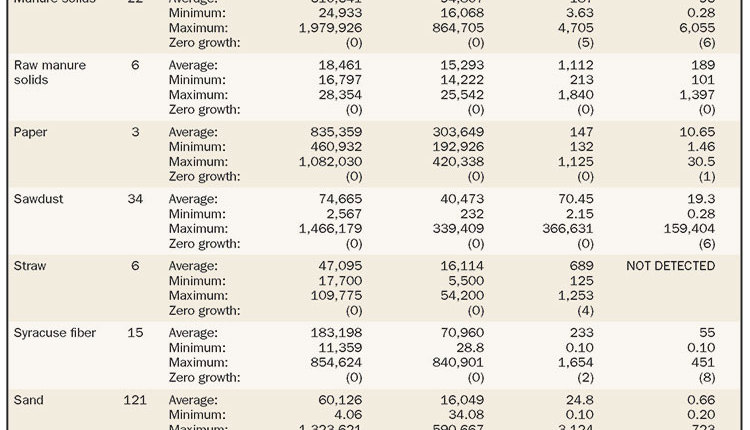
Eight of the participating dairy farms milking between 1,100 and 2,000 cows were monitored for clinical mastitis events from May 2016 to May 2017. The average SCC (somatic cell count) in these farms was 234,000 cells per milliliter (mL), ranging from 145,000 to 360,000 cells per mL. Five of the eight were below 200,000 cells per mL. The average milk per cow per day was 90 pounds, ranging from 86 to 96 pounds.
Milk samples were collected from every single cow with detectable signs of clinical mastitis. These on-farm samples were submitted for aerobic culture followed by precise microorganism identification using the technique of matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS).
A mastitis case was considered new if there was at least 14 days between a previously diagnosed case and the current case in the same quarter. The same held true if a different pathogen was identified. The monthly incidence risk at the cow level within herds was calculated each month as the number of clinical mastitis cases divided by the number of cows at risk.
What was found?
A total of 7,513 clinical cases were recorded during the year. The most common result was negative culture; this is likely due to the cow’s immune system affecting the bacteria’s ability to grow (killing it) such that there are no bacteria left in the milk sample at the time of clinical signs. The next most common result was environmental pathogens, especially coliforms (E. coli and Klebsiella spp.) and Streptococcus spp. In fact, Staphylococcus aureus was the only contagious pathogen isolated.
The most consistent finding among the eight individual farms was no growth that ranged from 20 percent to 40 percent of submitted samples for each farm. Gram-positive microorganisms caused a third of clinical cases among all farms (ranging from 17.1 to 37.1 percent); while gram-negative pathogens were identified in 26.3 percent of samples (ranging from 18.8 to 30.9 percent). All farms except one had at least a few cases of nonbacterial microorganisms such as yeast and/or Prototheca spp.
On Farms A, D, E, and G, E. coli was the most common pathogen isolated between 19 and 32 percent of the time. Klebsiella spp. showed a higher number of cases on Farms C and F (19 and 10 percent, respectively); while it was lowest on farm H (1 percent). Streptococcus uberis was an important pathogen on Farm C, F and H (34 percent, 21 percent and 16 percent, respectively) and the lowest on Farm A (1 percent). On Farm E, Staphylococcus aureus was the third most common cause of clinical mastitis with 10 percent of cases; whereas on Farm F and H, there were no cases due to this pathogen.
Other pathogens found included Enterococcus, Prototheca, Serratia, Pseudomonas, Streptococcus spp, Staphylococcus spp. T. pyogenes, Pasteurella, and gram-positive bacillus. These pathogens ranged from 9 to 19 percent across the farms.
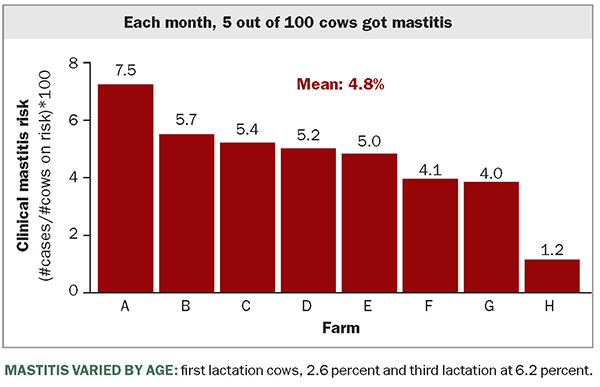
Over the study period, cow-level incidence risk for clinical mastitis was 4.8 percent, while the median was 5.1 percent. This means that out of 100 cows at risk for mastitis during each month, about five of them will experience a new case of clinical mastitis. As we look across these and other herds, this risk is unfortunately common.
The risk of clinical mastitis was 2.6 percent in first-lactation cows, while it was 4.7 percent, 6.2 percent, and 7.7 percent for cows in second, third, and four or more lactations, respectively. Therefore, as the lactation number climbs, the greater the chances for cows to experience a new clinical mastitis case.
The majority of mastitis occurred in the first 100 days in milk or DIM (53.4 percent), followed by 100 to 200 (32.3 percent), and lastly, greater than 201 DIM (14.3 percent).
More than 95 percent of cows with clinical mastitis during the study period had only one affected quarter at one time. No difference was observed in the distribution of clinical mastitis according to the quarter position: 49.7 percent of cases were identified in the front quarters while 50.3 percent affected rear quarters. Similarly, no differences were observed according to quarter sides: right hind was 25.8 percent; right front, 26.2 percent; left hind, 24.5 percent; and left front, 23.5 percent.
Make better decisions
This data provides important insights. The variation among the eight farms’ mastitis pathogens could be explained with specific farm information, such as udder health programs, milking routine, bedding management, housing conditions, cow comfort, culling decisions, and other variables.
When timely and accurate pathogen identification is provided, more precise treatment protocols can be followed on-farm. This leads to more economical use of antibiotics and more saleable milk by not treating cases where there are no bacteria present at the time of clinical detection.
As farms do a better job identifying mastitis cases and performing better milking procedures, the frequency of cows affected with contagious pathogens has diminished. Conversely, environmental microorganisms have emerged as the current challenge of mastitis control.
Utopia does not exist
It is practically impossible to eradicate environmental pathogens from dairy herds, especially because they survive and multiply in extramammary reservoirs. This data highlights the fact that although farms may have similar clinical mastitis incidence risk, the pathogen distribution was different between farms, thus practices to control and treat these mastitis causing pathogens may differ between farms.