The authors are a graduate student and a professor at the University of Calgary. Co-contributors include Kayley D. McCubbin, Julia Bodaneze, Ben Caddey, Waseem Shaukat, Diego Nobrega, Jeroen De Buck, and David Kelton.

Antibiotic resistance — why should you care?
Since the discovery of penicillin, many antibiotics have been introduced that allow us to treat all sorts of bacterial infections, benefiting human medicine immensely. However, antibiotic resistance is a growing problem, and 13,000 Canadians are dying each year of infections that can no longer be treated by antibiotics. In addition, more than 18,000 hospitalized patients per year acquire bacterial infections that are resistant to antibiotics. Without action, we might run out of options and find ourselves in a situation where common infections and minor injuries will be lethal again.
Misuse and overuse have led to the widespread occurrence of antibiotic resistance, especially inappropriate use in human medicine. However, use of antibiotics in animals should not be overlooked.
In animals, 1.8 times more antibiotics are used compared to humans (when corrected for differences in body weight). Additionally, a large portion of the antibiotics used in livestock overlap with those used to treat infections in humans. One should note, however, that the situation in Canada is not as dire as in other parts of the world. But that does not change the mounting pressure to reduce antibiotic use on livestock.
Studies in both human and veterinary medicine have indicated that when the amount of antibiotics is reduced, levels of antibiotic resistance also decrease. This effect was particularly present among people with direct contact with farm animals, such as farm workers and their families. The close proximity to their livestock elevates their risk to attract antibiotic-resistant bacteria, which is why focusing on antibiotic use in livestock is particularly important.
One might wonder why we cannot simply develop new antibiotics. Unfortunately, any new antibiotic will be reserved for hard to treat infections, such as tuberculosis. If we want to prevent a ban on antibiotics all together, we have to rely on reducing antibiotic use in order to cut back antibiotic resistance levels. For the dairy industry, this means that we need to focus on areas where a lot of antibiotics are used, which is at the start of the dry period in the form of dry cow therapy.
Be selective with therapy
For years, researchers advocated for blanket dry cow therapy in which all cows are treated with antibiotics at dry-off. However, over the last decades, the dairy industry has collectively improved udder health, as indicated by a low bulk tank somatic cell count (SCC). This has been possible due to improved milking hygiene, dry cow management, and availability of vaccines. As such, we have created a situation where we can better:
1. Identify the cows that have infections at dry-off.
2. Identify cows at risk of acquiring infections during the dry period.
3. Prevent infections during the dry period with the help of hygiene and teat sealants.
Therefore, most farms will be able to adopt a selective dry cow therapy strategy, where not all cows will receive antibiotics at the start of the dry period. The herds that would be fit to adopt a selective dry cow therapy protocol are those that have a bulk tank somatic cell count less than 250,000 cells/mL, excellent udder health management, good record keeping, and access to frequent SCC data. Subsequently, cows can be selected based on their SCC at dry-off and the occurrence of clinical mastitis in the current lactation (Figure 1). Teat sealants should be provided to those cows that do not receive any antibiotics.
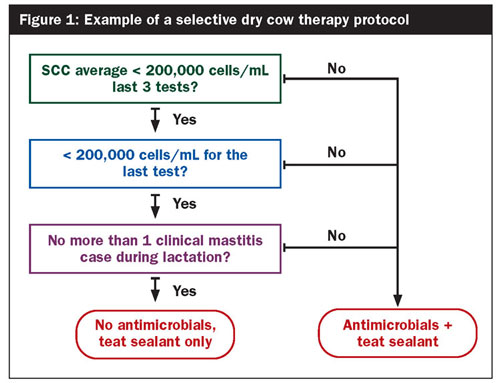
Selective dry cow therapy has been done in Scandinavia for decades. In addition, many studies have shown that when adopting such protocols on the right farms, there will be no negative consequences (such as risk of udder infection during the dry period or at calving or clinical mastitis in early lactation) when teat sealants are used instead.
The amount of antibiotics used to treat clinical mastitis cases can also be reduced on most dairy farms. This has been made possible by the availability of on-farm diagnostics tests and improved udder health. These tests can be used to identify cases that have active infections at the time of diagnosis. For some cases, infections have been cleared before clinical signs appear.
On-farm tests can also identify cases that are caused by the gram-positive bacteria Staphylococcus and Streptococcus. Gram-negative bacteria such as E. coli do not respond well to antibiotics, and clinical mastitis cases caused by gram-negative bacteria often clear up on their own, not requiring antibiotic treatment. In addition, chronic cases and cows with a history of clinical mastitis or subclinical mastitis have a lower likelihood of cure, which should also be taken into consideration (Figure 2).
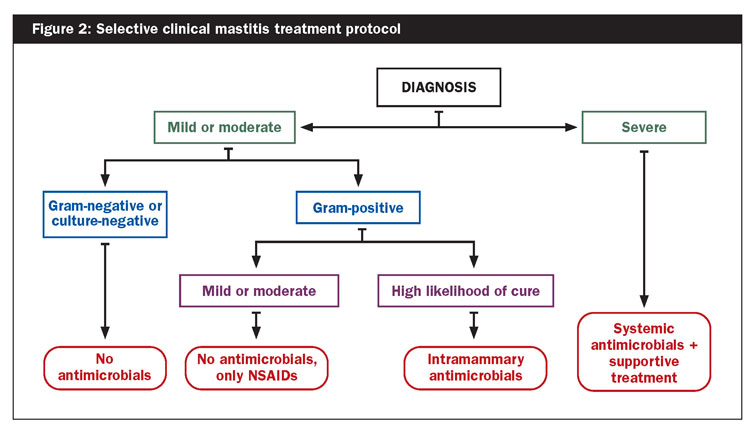
Studies have indicated that awaiting the results of the diagnostic tests and only treating gram-positive cases with antibiotics does not lead to any negative consequences — no changes in clinical cure rate, risk of new udder infections, recurrence, SCC, milk yield, or culling. As such, selective clinical mastitis treatment protocols can also be safely adopted on farms that practice good udder health management. For both dry cow and mastitis treatment protocols, hygiene and training of farm personnel is key.
2310_547