The author is the laboratory director for Udder Health Systems, Boise, Idaho.
Recently, I attended a herd health talk in which the presenter described a disease as the “silent thief,” and it struck me that, in my experience, Prototheca mastitis could be viewed analogously. This mastitis organism is often disregarded in lieu of more notable contagious pathogens, such as Staphylococcus aureus or Mycoplasma, and it may not even be part of the milk quality conversation at all.
Unlike other mastitis infection agents, Prototheca is a single-celled algae, not a bacteria. One reason it gets so little attention is that most infected animals are subclinical, with individual somatic cell counts (SCC) hovering around 200,000 to 400,000 cells per milliliter (mL) until late in the infection.
Slow developing situation
Reaching the point of elevated SCC and clinical mastitis may take multiple lactations, and many animals never show clinical mastitis at all. However, this situation does not mean they do not present risk to the herd during this period.
Historically, Prototheca has been thought of only as an environmental mastitis threat that could be eliminated by simply identifying and removing the source from the cow’s environment. More recent data, however, has now provided numerous instances of herd outbreaks, demonstrating cow-to-cow transmission can be a major driver of new infections.
Contagious spread of Prototheca may move slower than Staphylococcus aureus or Mycoplasma. But, like these pathogens, Prototheca will eventually reach a “tipping point,” in which the number of infected cows becomes incompatible with maintaining good milk quality and udder health in the herd.
Detecting the problem
Prototheca mastitis has become more recognized in the last 10 years as a causative agent of both clinical and subclinical mastitis. Prototheca colonies are easily missed on standard blood agar culture. That’s because it is a slow-growing organism, often with low colony counts, and is easily overgrown by other bacteria on the plate or may be misidentified as nonaureus staphylococci.
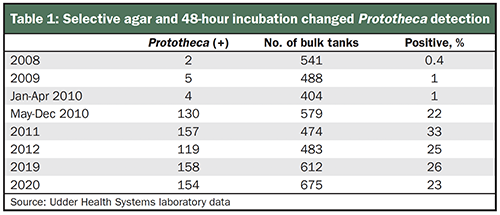
Detection can be greatly improved by use of a selective Prototheca agar and extending the incubation time to 48 hours at both the bulk tank and individual cow level. The Udder Health Systems laboratory routinely performs 400 to 600 comprehensive bulk tank cultures monthly from dairies located in Idaho, Montana, Oregon, and Washington.
Prior to the addition of this selective agar in 2010, less than 1% of the herds were bulk tank positive for Prototheca (Table 1). Detection of Prototheca climbed dramatically to 22% to 25% of the herds when incorporating a selective Prototheca agar into routine bulk tank culture screening after May 2010 as shown in Table 1.
When to look further
The presence of Prototheca in the bulk tank level should spur further investigation at the individual cow level. It means there are infected cows present in the herd that you should find, which may be difficult with blood agar alone. A review of 42,000 cow samples cultured on blood agar or gram-positive selective agar, alongside Prototheca agar, detected 2,252 Prototheca positives. Of the positive cultures, only 21% were detected on blood agar while 79% were identified on Prototheca agar (Table 2).
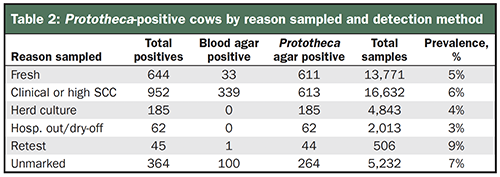
These samples either were submitted to Udder Health Systems as part of routine sampling or to investigate Prototheca-positive bulk tank cultures. In comparison to other contagious pathogens, prevalence of Prototheca may be mistakenly perceived to be very low, but the addition of enhanced detection methods may change the picture.
Environmental cultures are not standardized between laboratories and typically focus on streptococci and coliform counts; few, if any, will detect Prototheca without additional enhancement steps. The use of specific enhancement protocols on more than 300 environmental samples detected Prototheca in 40% of submitted samples from suspect herds.
Environmental sources of importance include standing water, manure, and bedding. Additionally, a critically important source of exposure is milking inflations contaminated by subclinical infections in herdmates.
In particular, recycled sand bedding and composted manure solids were problematic sources found to be a source of new infections. In contradiction to prior beliefs, it is rarely detected in water samples.
Control and prevention of Prototheca mastitis abides by the same guidelines as other contagious pathogens. Once infected cows are detected, there are two options:
• Permanent removal from the dairy cow herd
• Clear identification as a Prototheca cow (ear tag or leg band) and segregation to a pen where cows are milked last
Prototheca is unresponsive to antibiotic therapy and does not spontaneously cure. The infection process moves relatively slow, which allows the producer some time to make progress in getting in front of the spread.
It's a long journey
Eliminating Prototheca from a herd can be a long and frustrating process due to new infections from bedding or other environmental sources and infected animals shedding the organism in low numbers. Routine monitoring of all fresh cow cultures, as well as mastitis cows, for Prototheca will be a critical role in controlling the new infection rate. For dairies both large and small, utilizing results from an individual cow, bulk tank, and environmental cultures will allow them to make smart and economical management decisions in keeping Prototheca mastitis under control and eradicating it from the herd.






