The author is a professor and extension dairy specialist at the University of Idaho.
Many A.I. studs are now offering straws of semen that contain sperm from multiple bulls. To understand the potential advantage of a mixed, or “heterospermic,” A.I. dose, let’s review the journey of sperm from deposition to fertilization.
Following A.I. into the uterine body, sperm must travel to the site of fertilization at the midway point of the oviduct, known as the ampullary-isthmus junction. There are two phases of sperm transport. In the rapid phase, sperm reach the oviducts within minutes of deposition but are incapable of fertilization.
In contrast, sustained sperm transport requires six to 12 hours. Sperm traverse the uterine horns and utero-tubal junction to reach the lower portion of the oviducts (the isthmus). Here, they attach to the epithelium and form a reservoir.
Two of the most important functions of the sperm reservoir are to provide a favorable environment for capacitation and facilitate the release of groups of sperm, allowing a limited number to compete for fertilization. While millions of sperm are deposited with A.I., research has shown the majority of the inseminate is lost through retrograde flow, leaving only thousands in the oviducts 12 hours after insemination.
Capacitation is crucial
Capacitation involves the removal, addition, or modification of sperm plasma membrane components, and much of this occurs in the oviducts. Sperm capacitation is a prerequisite for fertilization and is thought to take about six hours. In conjunction with hyperactive motility and other molecular events, capacitation facilitates the release of sperm from the oviductal epithelium before fertilization.
Capacitated sperm make their way to the site of fertilization, where they undergo the acrosome reaction when contacting the outer covering of the egg, called the zona pellucida. The sperm acrosome is a cap-like structure that contains enzymes important for fertilization. Hyperactive motility is characterized by vigorous, high amplitude movement of the tail, and it is important for penetration of the zona pellucida.
Multiple sperm compete for fertilization. However, the first sperm to succeed induces the block to polyspermy, which stops other sperm from penetrating the zona pellucida. This ensures that there will be DNA from only one sperm in a resulting embryo, as polyspermy (DNA from multiple sperm) is lethal.
The sperm in an ejaculate, and therefore in A.I. doses, are incredibly diverse. Some sperm carry an X chromosome, while others carry a Y; some contain morphological differences, while still others may deviate in motility pattern. Some may capacitate at a different rate than other sperm for unknown reasons.
Some potential benefit
To exploit the heterogeneity of sperm in an A.I. dose, sperm from different males may be mixed. In research with rabbits, mice, cattle, swine, and birds, heterospermic inseminations with semen from two or more males (mixed on an equal basis) frequently resulted in a disproportionate number of offspring sired by one male. Due to the numerous factors contributing to the heterogeneity of ejaculates and A.I. doses within and across males, the reason for this phenomenon is unknown. Nevertheless, heterospermic A.I. may allow for desired fertility across a wider ovulation window, perhaps due to early and late capacitating sperm within the dose.
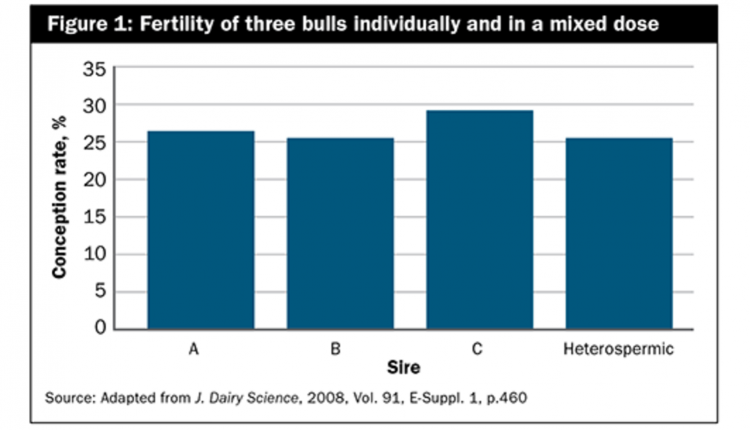
A study by an A.I. stud investigated the effects of heterospermic A.I. on fertility in lactating Holstein cows. Three Holstein sires with similar, above average fertility were used. Semen from each of the three bulls was collected, and three homospermic doses plus a heterospermic dose were frozen with 40 million sperm per dose. A.I. personnel were blind to the treatments.
Following 3,384 A.I. across five herds, conception rates for the homospermic doses and heterospermic mix were not different (Figure 1). Results from another study by the same A.I. stud using five bulls and more than 1,800 inseminations (with all A.I. doses at 20 million sperm per straw) agree, as the conception rates of the heterospermic doses were not different compared to fertility of each bull used singly.
Similar results in cattle and swine were found previously. Further, there is no evidence in these or other studies that the conception rate of a heterospermic mix will be greater than what is obtained from homospermic use of the highest fertility bull in the mixture. Rather, the benefit of heterospermic inseminations appears to be the minimization of poor conception rates should a lower fertility sire be selected. Happy A.I. breeding!









