The information below has been supplied by dairy marketers and other industry organizations. It has not been edited, verified or endorsed by Hoard’s Dairyman.
NovaVive Inc., an animal health immunobiology company, today announced that an abstract being presented at the 2021 International Embryo Technology Society (IETS) Virtual Conference (Jan. 18-21, 2021) is now available online in the journal, Reproduction, Fertility and Development. The abstract summarizes a research study evaluating the use of mycobacterium cell wall fraction (MCWF) (Amplimune ®) in heifer embryo transfer recipients. The research was led by Dr. J. Manuel Palomino, reproductive specialist at Boviteq Inc.
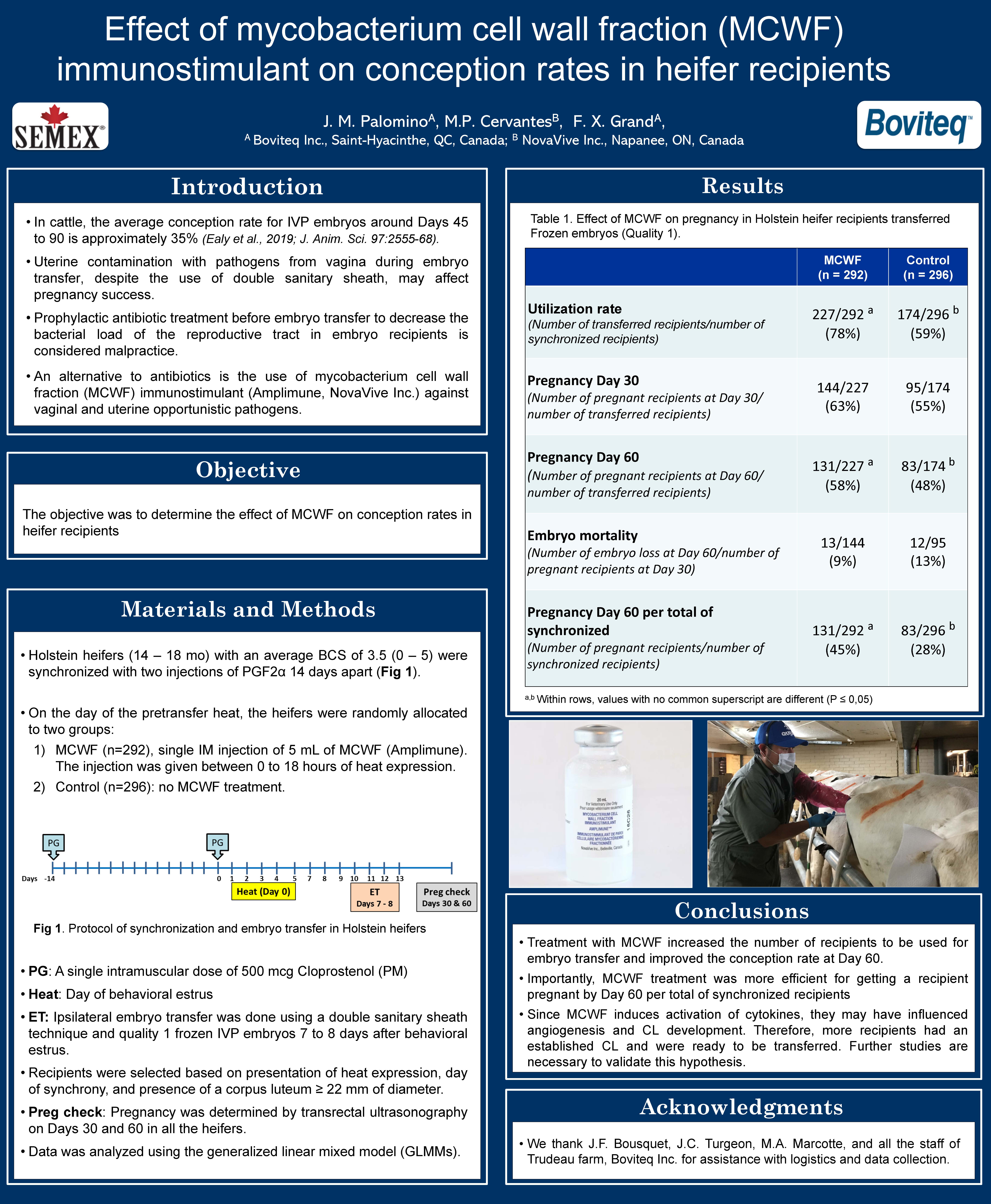
The objective of Dr. Palomino’s research study was to determine the effect of MCWF on pregnancy success in heifer recipients. Synchronized Holstein heifers were divided to be treated either with a single IM injection of Amplimune on the day of heat (MCWF group, n=292) or with no Amplimune (Control group, n=296). Frozen in vitro produced embryos were transferred 7-8 days later.
The researchers found that pregnancy per embryo transfer (P/ET) at Day 60 was higher (P<0.05) in the Amplimune group compared to the Control group (58% vs. 48%, respectively). In addition, they found that 78% of all synchronized heifers in the Amplimune group (vs. 59% in the Control group) were suitable to receive an embryo at the day of transfer, and received an embryo. As a result, pregnancy per synchronized recipient at Day 60 was greatly improved in the Amplimune group, with 131 of 292 heifers pregnant (45%) as compared to the Control group, with 83 of 296 heifers pregnant (28%).
The researchers concluded that treatment with MCWF at the day of heat increased the number of recipients to be used for embryo transfer and improved the P/ET at Day 60. They also noted that MCWF treatment greatly increased the number of recipients receiving embryos and, therefore, was a much more efficient approach to having a recipient heifer pregnant at Day 60.
The worldwide production of cattle embryos has increased during the last two decades from ~40,000 in 1997 to ~1,029,400 in 2018. In North America, there were 501,381 bovine embryo transfers in 2018 ( www.iets.org). However, pregnancy success in embryo transfer recipients remains sub-optimal. The average pregnancy success for in vitro produced embryos from Days 45 to 90 is 35%. Different factors may affect pregnancy success in an embryo transfer program, including the contamination of the uterine environment with pathogens during the embryo transfer process. Importantly, the use of antibiotics as a prophylactic measure in this situation is considered malpractice, therefore, alternatives to antibiotics are needed.
The recipients play a critical role in the success of ET programs. The anticipated value of using MCWF for embryo transfer is the improvement of pregnancy in heifer recipients which will help to maximize the efficiency of an embryo transfer system. The Company has assessed the value of embryos resulting in pregnancies, determining that the cost per pregnancy in the MCWF-treated heifers was approximately $111.45 versus a cost of $178.30 per heifer in the Control group.
About Amplimune
Amplimune is approved by regulators in the U.S., Canada, New Zealand and the United Arab Emirates to reduce the clinical signs and mortality associated with E. coli K99 diarrhea in neonatal calves. The product is an emulsion of mycobacterium cell wall fractions (MCWF) that enhances innate immunity to fight bacterial infections without the use of antibiotics. When injected into the animal, Amplimune enhances both innate and adaptive immune responses to fight bacterial infections. Amplimune is OMRI listed in the U.S. and Canada for use in organic production.
About NovaVive Inc.
NovaVive is a private company founded in July, 2014. The Company has an advanced veterinary immunotherapeutic platform based on mycobacterium cell wall fraction (MCWF) technology with 5 regulator-approved products in the U.S. (three of these are regulator-approved in Canada; two in Australia; and now three in New Zealand). Certain MCWF formulations have demonstrated the capability of reducing the reliance on antibiotics in the treatment of bacterial diseases of cattle and horses or effectively treating viral equine respiratory disease. Other formulations have been developed as anticancer therapies in dogs and horses. The Company’s development plan is to identify additional livestock and companion animal diseases that may be effectively treated with its immunotherapeutic technology platform. For more information about the Company, please visit www.NovaVive.ca









