The author is director, Veterinary Medicine Extension, Washington State University, Pullman. 
During an investigation at a large calf ranch, we had the opportunity to look at different measures of milk quality. We evaluated bacteria counts (like a bulk tank count) both pre- and postpasteurization, cell counts (SCC), pH (acidity), performed the alcohol test for coagulation, and evaluated total solids (nutrient content) in the waste milk using a Brix, hand-held refractometer.
The Brix refractometer we used measured total solids in the 5 to 15 percent range. We compared Brix total solids readings with spectrophotometry estimates of a large number of milk samples (some with added water) at a milk quality laboratory.
If we read 8 percent total solids on the refractometer, that meant we actually had about 10 percent total solids in the milk sample. We then looked at the waste milk from 12 dairies and found that some of them were very low in total solids, including the pooled milk. This told us that the calves were not getting the nutrients we expected they would from whole milk . . . normally at 12.5 to 13 percent total solids. The most likely way to have low solids is through dilution of the waste milk with water.
Next, we wanted to assess bacterial load. Most of the waste milk samples had very large numbers of bacteria, but pasteurization appeared effective at greatly reducing the number. What we don't know, however, is what effect ingestion of large amounts of dead bacteria has on baby calves. We can speculate from studies done in rats that it might be harmful because of high endotoxin levels from the death of coliform bacteria, but this has not yet been shown in calves.
High coliform levels could lead to high endotoxin levels in the milk. The wall of the intestine normally acts as a barrier to passage of endotoxin into the blood, but disruption of that barrier from intestinal infections or other causes could lead to systemic endotoxemia. However, many producers feed pasteurized waste milk successfully.
Many large calf ranches and dairies use blood agar plates or Petri film to assess bacterial counts after pasteurization. Keeping plates and an incubator going may not be for everyone, so you may want to have your veterinarian or milk quality lab do this for you.
The easiest way to evaluate milk spoilage appears to be estimating the pH of the milk using a handheld pH meter. The pH of milk will drop initially and then rise, depending on the stage of spoilage, time, temperature, and the types and number of bacteria present. Spoilage can affect not only color and odor which could affect intake but also can affect the nutrient content.
In our investigation, pH was correlated with the percent of total solids. The lower the pH, the lower the total solids measurement. Although intentionally acidified milk (such as by adding organic acids) has been fed to calves successfully, feeding spontaneously acidified (spoiled) milk may not have the same effects. Normal milk pH is about 6.5. Out of our 12 samples, we had five with pH less than 6.1 which may explain some of the low total solids.
The alcohol test is another test for spoilage. Also called the Ethanol Coagulation Test, it involves mixing equal amounts of milk and 70 to 75 percent alcohol. Coagulation is defined as fine particles of curd visible in the milk. The alcohol test did not appear to determine milk spoilage as well as pH did in our experience.
The somatic cell counts of our samples were all very high, since the milk came from hospital pens on the dairies. Monitoring SCC may not be a cost-effective way to assess milk quality. However, high somatic cell counts are associated with low total milk solids and low milk protein and may have contributed to the low total solids seen in some of our samples.
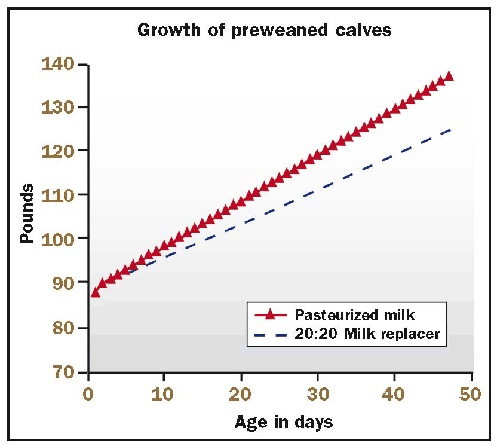
Whole milk will outperform standard milk replacers which have 20 percent protein and 20 percent fat on a dry matter basis. Whole milk runs about 27 percent crude protein and about 29 percent fat.
Our advice to you
There appears to be some easy ways to monitor the waste milk being fed to calves.
• Identify spoilage by using a pH meter. Discard milk with a pH less than 6.3 or greater than 7.0
• Identify the total solids in the waste milk. If the milk is not spoiled, but may have been diluted with water, estimate the total solids of the milk to be pasteurized and consider adding solids using a milk replacer powder.
• Periodically, assess the total bacteria count of pooled milk both before and after pasteurization. That's the only way to make sure the pasteurizer is functioning properly.
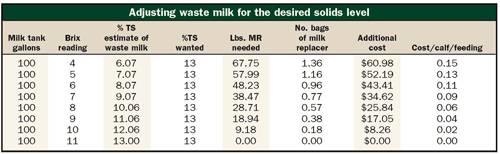
To help ensure that young calves get the nutrients they need, we set up a chart for the calf feeders on the calf ranch. See the table.
The calf feeder needs to first place the waste milk sample on the Brix refractometer and read the total solids percentage. When using the refractometer with waste milk, you need to clean the lens with alcohol to remove any fat residue left from previous samples, or the lines you see will appear very fuzzy. Our conversion table may work only for the Brix refractometer. We used (Reichert Brix 35HP which reads in the range of total solids for milk).
After getting a reading, you then can go to the chart and read the column on "%TS estimate of waste milk" to find the "actual" content. If the desired total solids level is 13 percent, and the milk is in a 100-gallon tank, and the Brix reading is 8 percent, the feeder will need to add about 29 pounds of milk replacer powder to the waste milk in the tank based on a standard 20 percent crude protein:20 percent crude fat milk replacer.
The additional costs given in the other columns are based on a 50-pound bag of milk replacer costing about $45. There may be a cost breakpoint at which you would want to go back to try to tighten up the waste milk handling procedures such as dilution with water rather than add milk replacer powder.
Click here to return to the Nutrition E-Sources
100425_315

During an investigation at a large calf ranch, we had the opportunity to look at different measures of milk quality. We evaluated bacteria counts (like a bulk tank count) both pre- and postpasteurization, cell counts (SCC), pH (acidity), performed the alcohol test for coagulation, and evaluated total solids (nutrient content) in the waste milk using a Brix, hand-held refractometer.
The Brix refractometer we used measured total solids in the 5 to 15 percent range. We compared Brix total solids readings with spectrophotometry estimates of a large number of milk samples (some with added water) at a milk quality laboratory.
If we read 8 percent total solids on the refractometer, that meant we actually had about 10 percent total solids in the milk sample. We then looked at the waste milk from 12 dairies and found that some of them were very low in total solids, including the pooled milk. This told us that the calves were not getting the nutrients we expected they would from whole milk . . . normally at 12.5 to 13 percent total solids. The most likely way to have low solids is through dilution of the waste milk with water.
Next, we wanted to assess bacterial load. Most of the waste milk samples had very large numbers of bacteria, but pasteurization appeared effective at greatly reducing the number. What we don't know, however, is what effect ingestion of large amounts of dead bacteria has on baby calves. We can speculate from studies done in rats that it might be harmful because of high endotoxin levels from the death of coliform bacteria, but this has not yet been shown in calves.
High coliform levels could lead to high endotoxin levels in the milk. The wall of the intestine normally acts as a barrier to passage of endotoxin into the blood, but disruption of that barrier from intestinal infections or other causes could lead to systemic endotoxemia. However, many producers feed pasteurized waste milk successfully.
Many large calf ranches and dairies use blood agar plates or Petri film to assess bacterial counts after pasteurization. Keeping plates and an incubator going may not be for everyone, so you may want to have your veterinarian or milk quality lab do this for you.
The easiest way to evaluate milk spoilage appears to be estimating the pH of the milk using a handheld pH meter. The pH of milk will drop initially and then rise, depending on the stage of spoilage, time, temperature, and the types and number of bacteria present. Spoilage can affect not only color and odor which could affect intake but also can affect the nutrient content.
In our investigation, pH was correlated with the percent of total solids. The lower the pH, the lower the total solids measurement. Although intentionally acidified milk (such as by adding organic acids) has been fed to calves successfully, feeding spontaneously acidified (spoiled) milk may not have the same effects. Normal milk pH is about 6.5. Out of our 12 samples, we had five with pH less than 6.1 which may explain some of the low total solids.
The alcohol test is another test for spoilage. Also called the Ethanol Coagulation Test, it involves mixing equal amounts of milk and 70 to 75 percent alcohol. Coagulation is defined as fine particles of curd visible in the milk. The alcohol test did not appear to determine milk spoilage as well as pH did in our experience.
The somatic cell counts of our samples were all very high, since the milk came from hospital pens on the dairies. Monitoring SCC may not be a cost-effective way to assess milk quality. However, high somatic cell counts are associated with low total milk solids and low milk protein and may have contributed to the low total solids seen in some of our samples.

Our advice to you
There appears to be some easy ways to monitor the waste milk being fed to calves.
• Identify spoilage by using a pH meter. Discard milk with a pH less than 6.3 or greater than 7.0
• Identify the total solids in the waste milk. If the milk is not spoiled, but may have been diluted with water, estimate the total solids of the milk to be pasteurized and consider adding solids using a milk replacer powder.
• Periodically, assess the total bacteria count of pooled milk both before and after pasteurization. That's the only way to make sure the pasteurizer is functioning properly.
To help ensure that young calves get the nutrients they need, we set up a chart for the calf feeders on the calf ranch. See the table.
The calf feeder needs to first place the waste milk sample on the Brix refractometer and read the total solids percentage. When using the refractometer with waste milk, you need to clean the lens with alcohol to remove any fat residue left from previous samples, or the lines you see will appear very fuzzy. Our conversion table may work only for the Brix refractometer. We used (Reichert Brix 35HP which reads in the range of total solids for milk).
After getting a reading, you then can go to the chart and read the column on "%TS estimate of waste milk" to find the "actual" content. If the desired total solids level is 13 percent, and the milk is in a 100-gallon tank, and the Brix reading is 8 percent, the feeder will need to add about 29 pounds of milk replacer powder to the waste milk in the tank based on a standard 20 percent crude protein:20 percent crude fat milk replacer.
The additional costs given in the other columns are based on a 50-pound bag of milk replacer costing about $45. There may be a cost breakpoint at which you would want to go back to try to tighten up the waste milk handling procedures such as dilution with water rather than add milk replacer powder.

100425_315







