The authors are assistant professor, Department of Animal Sciences, North Dakota State University, and Beef Quality Assurance Specialist, North Dakota State University, Fargo. This article is part of a series being prepared in cooperation with the American Association of Bovine Practitioners.

We're sure you think about the safety and quality of the milk you produce every day. Of course, milk is not the only food produced on dairy farms. You should be just as conscious of beef quality as you are of milk quality.
In today's beef market, cull dairy cows become more than just hamburger. Recent surveys show that well over half of all beef from dairy cows is used by foodservice companies and supermarkets as value-added products such as deli roast beef and marinated beef products such as fajita meats, and steaks.
Cull dairy cows and bulls also are important to your financial health. According to the National Animal Health Monitoring System (NAHMS), income from the sale of market dairy cows can account for between 5 and 10 percent of a dairy farm's gross revenue.
Avoid residues
A critical element of producing quality beef is ensuring that treated cows do not have illegal levels of drugs (drug residues) in them at the time they are sent to market. These residues are most easily avoided by keeping good records, identifying treated cows clearly, and using drugs in the manner specified on the drug label and the package insert, and paying special attention to the drug dosage, route of administration, and required waiting period (withholding time). If cows are ill, it may be smart to extend the label withholding time, even if the label route of administration and dosage are used. Sick animals may not clear drugs as effectively as healthy ones, due to dehydration and depressed function of drug-metabolizing organs, especially the liver.
As discussed in the previous article in this series (March 25, 2010, issue, page 209), any deviation from the instructions on the drug label (package insert) is called extra-label drug use and, by law, must be done only under the supervision of a veterinarian. Extra-label drug use usually requires longer withholding times. Your veterinarian will determine how much additional time must be added.
In cull dairy cows, the most common residue is caused by penicillin. However, flunixin is quickly moving its way to the top of the list.
Unfortunately, the incidence of residues in dairy cattle continues to rise. The number has gone from 727 in 2005 to 888 in 2006 to 1,001 in 2007 to 1,086 in 2008. (2009 numbers aren't available.) In 2008, 82 beef cows had residues, and the trend is downward.
There are several probable reasons why residues from dairy cows are on the rise. In order to understand why we are seeing more residues, we have to understand why an animal (and its carcass) would be tested.
Within a packing plant, a public health veterinarian conducts "inspector-generated sampling." This sampling occurs when the veterinarian suspects an animal may have an illegal (or violative) level of a drug residue.
Inspector-generated testing is directed at individual animals and at certain populations of animals. Individual animals that are targeted are those with lesions found at injection sites (the back leg) and those with active infections that reasonably could be suspected as recently being treated. Because of their higher incidence of residues, far more dairy cows were subjected to "inspector-generated testing" (80,131 head) than beef cows (4,678).
Populations of animals that are tested are those from farms with a history of residue violations. So, if you market one cow that is found to have illegal levels of drugs in her, cows you market in the future will be subjected to greater scrutiny. The USDA calls these populations of animals from farms with a history of residues animals from a "Same Source Supplier."
The USDA Food Safety Inspection Service (USDA-FSIS) has instructed the plant public health veterinarians to "increase testing of animals that the establishment receives from this same source supplier" and "to consider increasing scrutiny and testing of animals from a particular supplier when they have knowledge of findings of residue violations in animals from that supplier." To reduce the possibility of having a cow from your farm with a violative drug residue at slaughter, in addition to observing appropriate preslaughter withdrawal times, you should not market cows that are lame, sick, or recovering from recent surgery.
When a drug residue is found in an animal/carcass, the tissue the drug residue was found in always is condemned. Sometimes the entire carcass is condemned. The penalty for the residue is two-fold. The person who supplied the animal to the packing plant does not get paid, and the packing plant is unable to sell the carcass or tissue. If a dairy operation supplies more than one animal with a drug residue to the packing plant in a 12-month period, the dairy will be listed on the USDA-FSIS Same Source Supplier List. When a farm is added to this list, livestock from that farm will undergo more scrutiny and testing when marketed and harvested, and there is a possibility that the farm will not be able to market cull dairy cows. The potential negative impact to a farm's bottom line is huge.
What you can do
Here are a few additional quality assurance tips to help you improve the price you receive for your culled cows as well as the quality and safety of beef from your cows.
You can find the "Same Source Supplier List" at the USDA-FSIS website: http://origin-www.fsis.usda.gov/Science/Chemistry/index.asp. Click on "Same Source Supplier-Residue Violator List." The list is updated weekly.
Click here to return to the Animal Care E-Sources
100410_249

We're sure you think about the safety and quality of the milk you produce every day. Of course, milk is not the only food produced on dairy farms. You should be just as conscious of beef quality as you are of milk quality.
In today's beef market, cull dairy cows become more than just hamburger. Recent surveys show that well over half of all beef from dairy cows is used by foodservice companies and supermarkets as value-added products such as deli roast beef and marinated beef products such as fajita meats, and steaks.
Cull dairy cows and bulls also are important to your financial health. According to the National Animal Health Monitoring System (NAHMS), income from the sale of market dairy cows can account for between 5 and 10 percent of a dairy farm's gross revenue.
Avoid residues
A critical element of producing quality beef is ensuring that treated cows do not have illegal levels of drugs (drug residues) in them at the time they are sent to market. These residues are most easily avoided by keeping good records, identifying treated cows clearly, and using drugs in the manner specified on the drug label and the package insert, and paying special attention to the drug dosage, route of administration, and required waiting period (withholding time). If cows are ill, it may be smart to extend the label withholding time, even if the label route of administration and dosage are used. Sick animals may not clear drugs as effectively as healthy ones, due to dehydration and depressed function of drug-metabolizing organs, especially the liver.
As discussed in the previous article in this series (March 25, 2010, issue, page 209), any deviation from the instructions on the drug label (package insert) is called extra-label drug use and, by law, must be done only under the supervision of a veterinarian. Extra-label drug use usually requires longer withholding times. Your veterinarian will determine how much additional time must be added.
In cull dairy cows, the most common residue is caused by penicillin. However, flunixin is quickly moving its way to the top of the list.
Unfortunately, the incidence of residues in dairy cattle continues to rise. The number has gone from 727 in 2005 to 888 in 2006 to 1,001 in 2007 to 1,086 in 2008. (2009 numbers aren't available.) In 2008, 82 beef cows had residues, and the trend is downward.

There are several probable reasons why residues from dairy cows are on the rise. In order to understand why we are seeing more residues, we have to understand why an animal (and its carcass) would be tested.
Within a packing plant, a public health veterinarian conducts "inspector-generated sampling." This sampling occurs when the veterinarian suspects an animal may have an illegal (or violative) level of a drug residue.
Inspector-generated testing is directed at individual animals and at certain populations of animals. Individual animals that are targeted are those with lesions found at injection sites (the back leg) and those with active infections that reasonably could be suspected as recently being treated. Because of their higher incidence of residues, far more dairy cows were subjected to "inspector-generated testing" (80,131 head) than beef cows (4,678).
Populations of animals that are tested are those from farms with a history of residue violations. So, if you market one cow that is found to have illegal levels of drugs in her, cows you market in the future will be subjected to greater scrutiny. The USDA calls these populations of animals from farms with a history of residues animals from a "Same Source Supplier."
The USDA Food Safety Inspection Service (USDA-FSIS) has instructed the plant public health veterinarians to "increase testing of animals that the establishment receives from this same source supplier" and "to consider increasing scrutiny and testing of animals from a particular supplier when they have knowledge of findings of residue violations in animals from that supplier." To reduce the possibility of having a cow from your farm with a violative drug residue at slaughter, in addition to observing appropriate preslaughter withdrawal times, you should not market cows that are lame, sick, or recovering from recent surgery.
When a drug residue is found in an animal/carcass, the tissue the drug residue was found in always is condemned. Sometimes the entire carcass is condemned. The penalty for the residue is two-fold. The person who supplied the animal to the packing plant does not get paid, and the packing plant is unable to sell the carcass or tissue. If a dairy operation supplies more than one animal with a drug residue to the packing plant in a 12-month period, the dairy will be listed on the USDA-FSIS Same Source Supplier List. When a farm is added to this list, livestock from that farm will undergo more scrutiny and testing when marketed and harvested, and there is a possibility that the farm will not be able to market cull dairy cows. The potential negative impact to a farm's bottom line is huge.
What you can do
Here are a few additional quality assurance tips to help you improve the price you receive for your culled cows as well as the quality and safety of beef from your cows.
- Only employ extra-label drug use when prescribed by a veterinarian who has a valid veterinary client-patient relationship with your herd.
- Review all treatment records to determine if the animal you are planning to market has met all withdrawal times.
- Avoid being an industry "black eye." Do not market any animals with lameness scores of 4 or higher. These cows are more apt to become nonambulatory animals (downers) in auction markets, on trucks, and in packing plants. Plus, these cows often are targeted for residue testing!
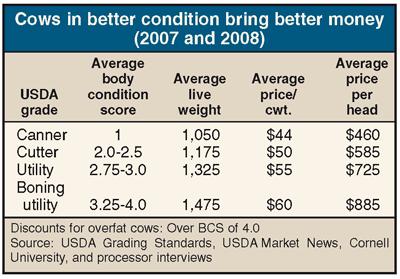
- Market cows with a body condition score of 2 or higher. (See table.) Research shows a live value difference of between $300 and $425 per head when comparing a body condition score of 2 to a score of 3.5.
You can find the "Same Source Supplier List" at the USDA-FSIS website: http://origin-www.fsis.usda.gov/Science/Chemistry/index.asp. Click on "Same Source Supplier-Residue Violator List." The list is updated weekly.
100410_249