Mastitis, the inflammation of one or more mammary glands, is one of the most important diseases in dairy production. Most mastitis cases are caused by intramammary infection, with pathogenic bacteria entering the mammary gland through the teat canal. Based on the presence or absence of clinical signs, mastitis can be differentiated into clinical and subclinical mastitis.
Clinical mastitis is defined when alterations in the milk or signs of inflammation of the affected quarter with or without systemic signs are present. Subclinical mastitis is characterized by an inflammatory response that is not reflected by visually detectable signs but only laboratory and cowside testing methods. These include elevated somatic cell count (SCC) and greater conductivity resulting from higher leukocytes and milk sodium and chloride concentrations.
Reducing antibiotic use
Because mastitis reduces milk production, reproductive performance, and longevity in the herd, it causes economic losses for the dairy producer. In addition, mastitis is considered to diminish animal well-being. Thus, much research has been done to improve treatment options for bovine mastitis to alleviate the aforementioned sequelae.
The principle of infusions of antimicrobial solutions into the mammary gland as means of mastitis treatment has existed for at least a century. To date, antimicrobial therapy continues to be the main strategy for mastitis treatment in dairy cattle, and mastitis was reported to account for most antimicrobial drug use in dairy herds. However, due to the emergence of antimicrobial resistance and its associated public health concerns, use of antimicrobials to treat livestock is rigorously scrutinized.
Antimicrobial treatment of subclinical mastitis is often omitted as compensatory production gains following therapy fail to outweigh treatment costs. Further, chronic intramammary infections, for example — those with pathogens that can reside intracellularly, form abscesses, and cause extensive fibrotic changes (such as Staphylococcus aureus) — are more difficult to combat. Lower cure rates have been reported compared with newly acquired intramammary infections.
One quarter at a time
Consequently, there is a demand for additional nonantimicrobial management strategies for treatment of subclinical mastitis and chronic intramammary infections. Premature agalactia or individual quarter dry-off (QDO) of infected quarters has been practiced as a treatment regimen for cows with recurrent mastitis and chronically elevated SCC.
There is anecdotal evidence that cows display compensatory milk production in three quarters like what is attained from four quarters. Another argument supporting QDO as a viable practice is the reduction of reservoir of infection, thereby reducing the risk of new infection for previously noninfected cows.
However, knowledge about the influence of QDO on milk production and SCC as well as risk of clinical mastitis and removal from the herd in conventional systems is scarce. The objectives of this study, therefore, were to investigate the influence of QDO on milk yield and SCC.
The study design
To achieve our goal, we used data from a commercial dairy farm where individual quarter dry-off has been used to manage cows with chronic mastitis and high SCC. Cows subjected to QDO were identified with a colored leg band indicating the position of the respective quarter. Milking technicians were instructed to not attach the milking unit to the respective quarter, and no antimicrobial or teat sealants were used.
We analyzed records from 471 dairy cows subjected to QDO from a 4,000-cow dairy with a 3x milking schedule. We randomly selected herdmates that were not subjected to QDO at a ratio of 1:1, balanced by parity and stage of lactation to serve as a comparison group (CON).
We used appropriate statistical methods to estimate milk yield and SCC one test day prior to (T -1), as well as one, two, and three (T 1 to T 3) test days after the event of QDO. In addition, we set out to study the risk of QDO on clinical mastitis occurrence and removal from the herd during the first 100 days after the QDO event had occurred.
Cows were in their first (138, 29%), second (132, 28%), and third or greater (201, 43%) lactation and between one and 491 days in milk (DIM) at the time of QDO (mean ± SD, 99 ± 85; median, 81). The frequency distribution of QDO among quarters was left front, 131 (28%); left hind, 98 (21%); right front, 123 (26%); and right hind, 119 (25%).
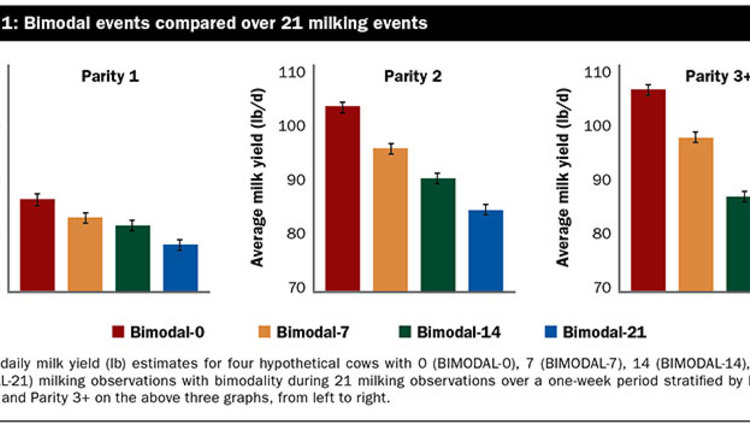
The average milk yield for both groups over the four test days was 91.5 pounds and ranged from 5.1 to 166.9 pounds per day. The average values at T -1, T 2, T 3, and T 4, respectively, were 79.6, 76.9, 77.6, and 74.1 pounds per day for cows in group QDO, and 90.4, 94.4, 91.9, and 86.6 pounds per day for cows in group CON (Figure 1).
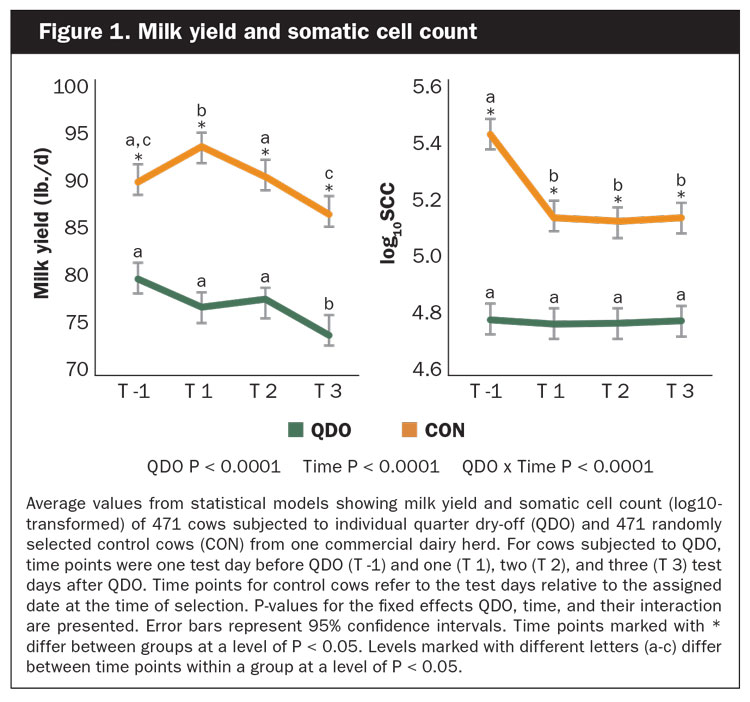
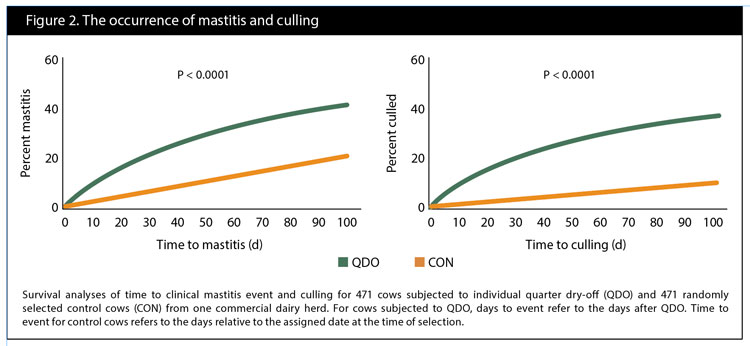
The median SCC for both groups over the four test days was 61,000 and ranged from 3,000 to 9,999,000 cells/mL. Average values for log10SCC at T -1, T 2, T 3, and T 4, respectively, were 5.4, 5.1, 5.1, and 5.1 for cows in group QDO, and 4.8, 4.8, 4.8, and 4.8 for cows in group CON, also shown in Figure 1. Survival analyses of the time to clinical mastitis and culling are depicted in Figure 2.
Other quarters compensated
We observed a numerical reduction in milk yield in three-quartered cows after the QDO event. However, this decline was not meaningful, suggesting compensatory milk production of the three remaining quarters. SCC fell after the QDO event; however, it did not reach the level of the CON cows. QDO cows had a higher risk of clinical mastitis and a higher risk of removal from the herd within the first 100 days after the QDO occurred.
We conclude that drying off one quarter can be a viable strategy to manage cows with chronically elevated somatic cell count and recurrent clinical mastitis. We warrant future research to inform best selection practices of cows that can be subjected to QDO and how we can reduce the risk of clinical mastitis of cows subjected to QDO and lower their risk of removal from the herd.