
On January 1, 2017, the Veterinary Feed Directive (VFD) will take effect. This FDA regulation will change the way that antibiotics considered medically important to human health can be accessed by farmers who produce animal-derived food (cattle, pigs, sheep, goats, fish, and so forth).
The purpose of the VFD is to have veterinarian oversight on medically important antibiotics used for treatment, prevention, and control of disease in livestock. It will also eliminate the use of these antibiotics for growth promotion and feed efficiency purposes.
In dairy production, the majority of the antibiotics affected by the VFD are used in calves and young stock. Two important changes are taking place. Many current over-the-counter (OTC) antibiotics put into feed will now require a VFD issued by a veterinarian who has a valid Veterinary-Client-Patient Relationship (VCPR) with the producer. Secondly, medically important water soluble OTC antibiotics will become prescription only. Products not affected include injectable antibiotics and feed grade products not used in human medicine, such as ionophores (Rumensin and Bovatec).
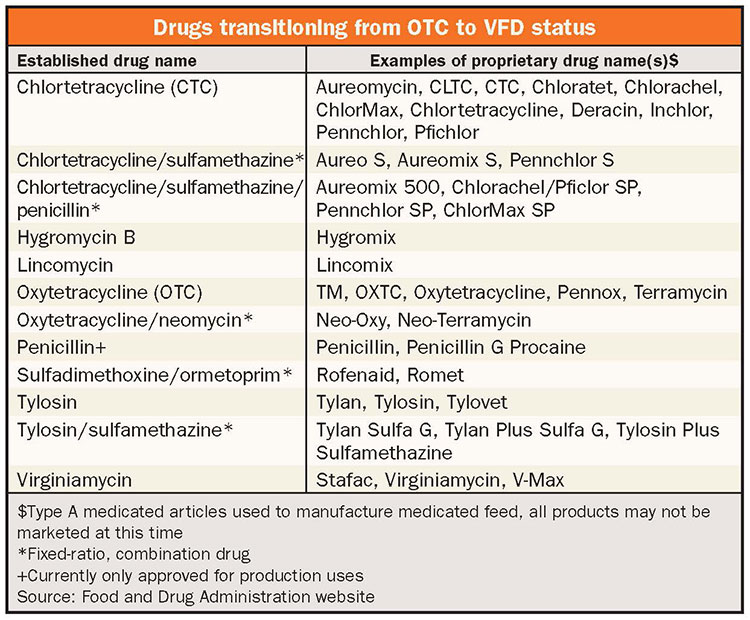
Examples of antibiotics used in young calves that will transition to VFD include chlortetracycline, oxytetracycline, and neomycin. For antibiotics requiring a VFD, extra-label use is not allowed. A veterinarian is not able to change the feeding directions (class of animal treated, treatment use, rates, or duration) of these medicated feeds. Another consideration is the use of combinations of drugs in feed (including ionophores) will be illegal without a specific approval for such use.
Make plans now
According to the USDA’s 2014 survey of Dairy Cattle Management Practices in the United States, some operations are using antibiotics delivered in feed for preweaned heifers. According to the survey, 1.6 percent of operations are using chlortetracycline, 4.9 percent are using oxytetracycline, and 9 percent are using a neomycin/oxytetracycline combination on preweaned heifers. Moving forward, these antibiotics will require a VFD from your veterinarian if you intend to continue delivering them through feed. They must only be fed for the duration approved on the product label.
To continue to use medicated milk replacers or starter feeds after January 2017, inquire now about future availability as manufacturers may change production, and some mills may decide not to carry medicated products at all. Please note, the majority of dairy calves are successfully raised without the use of antibiotics in feed. Now is a good time to work with your veterinarian and examine management changes that can reduce the use of antibiotics in feed.
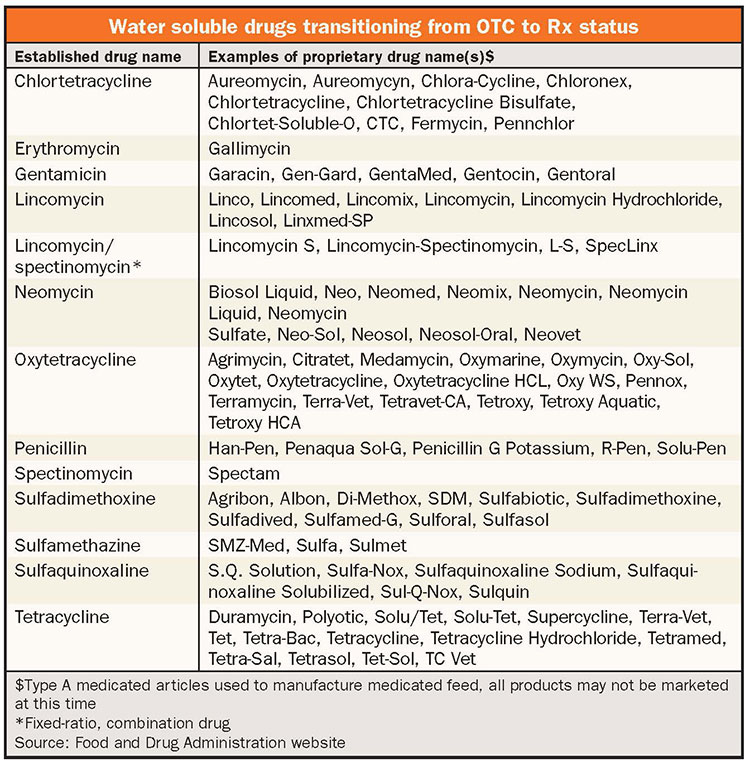
For water soluble drugs transitioning from OTC to prescription, the major change comes in the availability of the product. Products will require a prescription written by a veterinarian and will only be available through retail outlets that accept medical prescriptions. In addition, water soluble drugs may not be mixed into milk (or feed), even under veterinary direction.
Extra-label use under the direction of a veterinarian is still permitted for these drugs. For example, a veterinarian can instruct a producer to use a water soluble prescription antibiotic at a higher dosage than listed on the label, but they cannot instruct the producer to feed it in the milk. There is no current data on how much over-the-counter water soluble products are being used in preweaned calves; however, it is anecdotally known that significant use exists for products like lincomycin, neomycin, oxytetracycline, sulfamethazine, and tetracycline.
Plan ahead, and work with your veterinarian to discuss the antibiotic products you use in feed and milk so your veterinarian can develop a plan best suited to your farm’s needs. Now is the time to re-evaluate facilities and management practices that can reduce scours and respiratory disease so that your farm can have healthy calves with minimal antibiotic treatments.
This article appears on page 737 of the December 2016 issue of Hoard's Dairyman.











